2-5. ELECTROLYTES
The amounts of intracellular and extracellular fluids contained in a person’s body are extremely important to his healthy physiology. Losses of body fluids by vomiting, diarrhea, or excessive perspiration can produce illness or even death. Whenever body fluids are lost, the substances dissolved in the fluids are also lost. Certain inorganic substances are found in the body’s fluids. These are called “electrolytes.” Examples of electrolytes are potassium and chloride. These electrolytes exist in their “ion” state in the body fluids. This means that each ion can combine with one or more ions to form needed body compounds or can produce electro-chemical equilibrium (or balance). One example of this is the osmotic pressure that causes water to flow across a cell membrane. The relationship between the concentrations of sodium and potassium electrolytes in the cells and the extracellular fluid causes the water to flow into and out from the cells. There is usually a low level of sodium in the cells and a high concentration of potassium. The milliequivalent (mEq) is a unit of measure for the electrolyte.
a. The movement of electrolytes is governed by their electrical charge. Some are positively charged and are called “cations.” Others are negatively charged and called “anions.” Below are the major electrolytes, their chemical abbreviations, and the amount of each contained in a liter of extracellular fluid.
| Major Electrolytes and Amount found in 1 liter of extracellular fluid. | |
| Sodium (Na+) | 140 mEq |
| Chloride (Cl–) | 100 mEq |
| Bicarbonate (HCO3 –) | 27 mEq |
| Potassium (K+) | 4 mEq |
| Magnesium (Mg+2) | 3 mEq |
(1) Certain compounds can be formed by combining these charged cations and anions.
(a) To form magnesium chloride: Each ion of magnesium has two positive charges (Mg+2). There are 3 mEq of magnesium in each liter of extracellular fluid. Therefore, each liter of extracellular fluid has 6 positive ions of magnesium (+2 x 3 = +6) available to combine with other negative ions. If the compound, magnesium chloride is needed, these six positive ions could combine with six negative ions from the 100 mEq of chloride contained in a liter of extracellular fluid (each electrolyte of chloride has one negative charge, shown as Cl–). This would yield the required magnesium chloride by neutralizing the positive and negative ions.
(b) To form sodium bicarbonate: Each ion of sodium has one positive charge (Na+). There is one negative charge of bicarbonate (HCO3–) in each ion of bicarbonate. If the compound, sodium bicarbonate is required, each positive sodium ion can combine with a negative bicarbonate ion to yield the required amount of sodium bicarbonate. This neutralizes the positive and negative ions of both the sodium and the bicarbonate used
(2) When the body loses fluids, the number and kind of electrolyte(s) lost will depend on whether the fluid has been lost from interstitial or intravascular spaces or both.
b. Intracellular fluid contains the following kinds and amounts of electrolytes per liter:
| Major Electrolytes and Amount found in 1 liter of intracellular fluid. | |
| Potassium (K+) | 160 mEq |
| Phosphate (PO4–3) | 110 mEq |
| Magnesium (Mg+2) | 25 mEq |
| Sodium (Na+) | 5 mEq |
| Chloride (Cl–) | 3 mEq |
c. Each electrolyte has certain functions in order to help the body maintain homeostasis.
(1) Sodium. As you can see, sodium is the most abundant positive electrolyte (or cation) in the extracellular fluid and is also present in intracellular fluid. The main function of sodium is in maintaining normal osmotic pressure.
(2) Chloride. Chloride is the most abundant negative electrolyte (or anion) in extracellular fluid and is present in intracellular fluid as well. Chloride is essential to maintain normal osmotic pressure and is found in the stomach fluid.
(3) Potassium. Potassium is the most abundant electrolyte in the intracellular fluid. Potassium is also required for osmotic pressure but has other vital functions. Potassium is required to convert dextrose (a sugar) into body energy and is required as an aid in transmitting nerve impulses within the heart.
(4) Bicarbonate. Bicarbonate helps to maintain the acid-base balance within the body (see paragraph 2-6).
(5) Phosphate. Phosphate is required for the formation of bones, teeth, and body enzymes (see paragraph 2-10 for a discussion of enzymes).
(6) Magnesium. Magnesium is essential for the formation of enzymes within the body.
(7) Calcium. Calcium is essential for the formation of bones and teeth. Calcium is needed to help in blood clotting and in maintaining the rhythm of the heart beat
d. If a liter of body fluid is lost from extracellular fluids, all the electrolytes must be replaced along with the water. If a liter of fluid is lost by a severe case of diarrhea, the electrolytes would be of a different type and amount than those from the extracellular fluid. Therefore, both the source of the lost fluid and the amount must be considered when giving replacement fluids.
2-6. ACID-BASE BALANCE
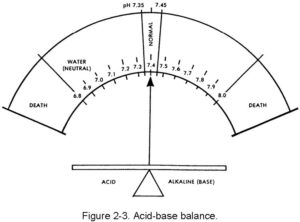
The body’s internal liquid environment is comprised of the body’s fluids and the blood. For proper body functions to continue normally, this internal environment must be kept constant (homeostasis) and within a very narrow limit. The acid-base balance of the blood is maintained by the chemical balance between the cations and the anions, which must be there in a very delicate balance. The cations are sodium (Na+), potassium (K+), calcium (Ca+2) and magnesium (Mg+2). The anions are chloride (Cl–), bicarbonate (HCO3–), phosphate (PO4–3), and sulfate (SO4–2). The acid-base balance is normally expressed as the “pH.” This expression is a relationship of the hydrogen ion (acid) concentration in the blood (or in the body) and an arbitrary number. The balancing part (base or alkaline) is a hydroxy group (OH –). The normal range of the blood pH is 7.35 to 7.45. There is a daily variation caused by production of acids by exercise and metabolism of food. In the terminal stages of some diseases, pH can vary from a low of 6.8 to a high of 7.8. The concept of pH may be easier to understand by comparing the acid (hydrogen) and base (hydroxl) factors to pure water, which is neutral (neither acid nor base). The body is always slightly alkaline. The body’s acid-base balance is effectively maintained under normal circumstances by the various buffer processes which neutralize strong acids or strong bases (alkalines) using the body’s various buffer systems (chemical, organic, and so forth) to help excrete excess body system products. Figure 2-3 shows the narrow range of the body’s pH.
2-7. NATURE OF SOLUTIONS
There are three movement directions possible following the introduction of injectable solutions into the body. These movement directions are governed by the nature of the solution with regard to body fluids. The fluids are called hypotonic, hypertonic, and isotonic.
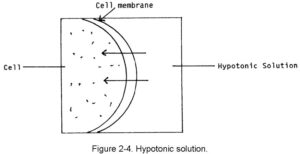
a. Hypotonic Solutions. A hypotonic solution is one that has less tonicity than the fluid within the body’s cells. This type of fluid is absorbed into the body’s cells by moving across the cell’s membrane and into the cell. If too much hypotonic solution is added, there is always the danger that the cells could burst or at least become irritated. Examples of hypotonic solutions are 0.45 percent sodium chloride solution and sterile water. This movement is shown in figure 2-4.
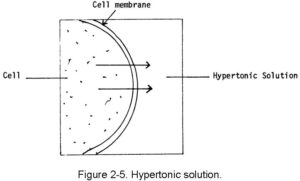
b. Hypertonic Solutions. A hypertonic solution is one that has greater tonicity than the fluid within the body’s cells. When this type of fluid is injected, it causes the cells to lose fluid into the surrounding spaces. If too much hypertonic solution is injected, the cells will shrink and shrivel. The cells become irritated, and this will probably cause pain at the site of administration. Examples of hypertonic solutions are hyperalimentation solutions and 10 percent dextrose solution. This movement is shown in figure 2-5.
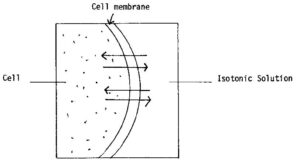
c. Isotonic Solutions. An isotonic solution has the same tonicity as that of body fluids. When this type of fluid is injected, fluids travel equally in both directions. Injection of an isotonic fluid causes no cell irritation to occur. Examples of isotonic fluids are 0.9 percent sodium chloride solution and lactated Ringer’s solution. This movement is shown in figure 2-6.
2-8. DIFFUSION AND OSMOSIS
Even though the predominate movement of hypotonic solutions and hypertonic solutions is in one direction, there is always a weak movement in the opposite direction. This weak movement is caused by diffusion. This process is in contrast to osmosis, which is a directional movement.
a. Diffusion. When a solute is added to a fluid, the molecules will begin immediately to spread throughout the fluid. This process is diffusion. The movement is random. It is the nature of molecules to move constantly in a fluid. Even though no special effort (like stirring) is made to mix the solution, the solute will be evenly distributed within a period. An illustration of diffusion is to add ice cubes to a warm liquid. Without stirring, the cold molecules will distribute themselves among the warmer ones. This will create a liquid of uniform temperature. The diffusion process is not limited by the presence of a semi-permeable membrane as long as the molecules are small enough to travel through the membrane. Cell walls are semi-permeable membranes.
b. Osmosis. The process of osmosis is unidirectional across a semi-permeable membrane. When the molecules of a solute are too large to travel through the membrane wall, they will remain on one side. When a solvent is added to the other side of the membrane, the molecules of the solvent will travel through the membrane to the side with the greater concentration of the solute. This is the principle by which the hypotonic and hypertonic solutions move fluid across the cell membranes.
2-9. CRYSTALLOID AND COLLOID SOLUTIONS
The nature of injectable crystalloid and colloid solutions determines their ability to be absorbed by the cells or to remain in the circulatory system.
a. Crystalloid Solutions. Crystalloid solutions contain small molecules that pass freely through cell membranes and vascular system walls. These solutions are useful as fluid expanders and are stored at room temperature. The crystalloid solutions are a useful source for electrolytes and a temporary source of fluid volume. They flow out of the vascular system rather quickly. Lactated Ringer’s is an example of a crystalloid solution.
b. Colloid Solutions. The colloid solution contains molecules that are frequently very complex and much larger than those in the crystalloid solutions. A solution that contains protein is colloidal. The colloidal solutions are needed when a solution is required to remain in the vascular system. Colloid solutions generally require refrigeration and can be stored for a limited period. Whole human blood U.S.P. and Hetastarch are examples of colloid solutions.
2-10. NORMAL FLUID LOSS
As mentioned earlier, there are four ways the normal healthy body loses fluids daily. People are generally unaware of the loss of fluids since the body does an excellent job of replacing the lost amounts of fluid and electrolytes. It is only when there is a severe loss during illness or injury that the body has difficulty replacing these losses.
a. Perspiration. Perspiration is a constant route for fluid loss. About 650 milliliters of perspiration are lost during a normal day by a healthy person. Along with water, a liter of perspiration has about 45 mEq of sodium, 4.5 mEq of potassium and 57.5 mEq of chloride. A normal person only becomes aware of the perspiration loss during very strenuous exercise or when the day is very hot. As a general rule, where sodium goes, water follows. That is why there is always sodium on the skin following heavy perspiration.
b. Respiration. Respiration occurs around-the-clock, but most people are not aware that they are exhaling fluid in the form of water vapor every time they breathe. We become aware of the vapor loss during cold weather when the vapor can be seen as it is exposed to low temperatures. About 450 milliliters of fluid are lost during a twenty-four hour period by respiration.
c. Feces. A small amount of fluid is normally contained in the feces. About 100 milliliters of fluid are lost in this manner during a day.
d. Urine. Urination accounts for the greatest fluid loss daily. About 1300 milliliters of urine are excreted by the normal person each day.
e. Total. If these four loss routes are totaled, we find that about 2500 milliliters of fluid are lost and must be replaced daily. See figure 2-7 for a summary.
|
Figure 2-7. Summary chart for normal fluid loss. |
|
| Perspiration | 650 ml |
| Feces | 100 ml |
| Respiration | 450 ml |
| Urination | 1300 ml |
| Perspiration | 650 ml |
| Total | 2500 ml daily |
2-11. ABNORMAL FLUID LOSS
There are seven means of abnormal fluid loss from the body, and these are generally quite apparent to the individual. Vomiting and diarrhea are the most frequent and obvious.
a. Vomiting. A person who experiences severe vomiting not only loses the fluids taken orally, he also loses the gastric fluids (or juices) that are secreted into the stomach. These juices are rich in electrolytes. For example, a liter of gastric juice contains about 50 mEq of bicarbonate.
b. Diarrhea. Diarrhea (loose, watery stools) frequently accompanies vomiting when people have “bugs” or the “flu.” Diarrhea is not only very uncomfortable and unpleasant, it accounts for a large loss of body fluids. A loss of electrolytes (sodium, potassium, chloride and others) is accompanied by the digested nutrients present in the diarrhea that is not absorbed by the body. For this reason, a severe bout of diarrhea is followed by general weakness.
c. Severe Perspiration. Severe perspiration that follows strenuous exercise in a hot environment can cause loss of electrolytes as well as heavy loss of fluid. Heat injuries can result.
d. Severe Burns. In very severe or widespread burns, the loss of protection by the skin allows body fluids to seep from the burned skin. This is a very serious effect of burns. The fluid and electrolytes must be replaced. Fluids and electrolytes can seep from the burns as fast as or faster than replacement fluids are administered.
e. Gastric Suction. Gastric suction of the stomach produces an effect similar to severe vomiting. Before long, the patient will lose a tremendous amount of electrolytes along with the fluids.
f. Bleeding. Bleeding of any amount produces loss of plasma, red blood cells, and other dissolved substances. The effect of the loss becomes greater as the amount of bleeding increases.
g. Diuretic Drugs. A person who is being treated with thiazide diuretics also loses fluids and electrolytes (primarily potassium). In such cases, potassium must be given to the patient to prevent potassium deficiencies.
2-12. REPLACING ABNORMAL FLUID LOSS
When fluid and electrolyte loss becomes abnormal, the medical specialist, the physician assistant, or the physician has two means of correcting the fluid and electrolyte balance–oral (by mouth) and intravenous.
a. Oral Route. The safest route is oral. For example, a person on thiazide diuretics may be given orange juice or tablets to replace the potassium needed. This is the preferred route since the patient is able to move freely and the psychological problems of intravenous administration are not present.
b. Intravenous Route. The intravenous route makes it possible to control the volume of fluid and the numbers of electrolytes to be given. Infection can be caused by a contaminated intravenous solution, administration set, or administration site. This will complicate the recovery of an already ill or injured patient. Another consideration is the total volume of fluid to be administered to the patient over a given time period.
Generally, an adult patient should not receive more than four liters (4,000 milliliters) of intravenous fluid over a 24-hour period. A careful record must be kept of the volume of fluid administered since this must be taken into account when calculating a patient’s fluid intake and output. The renal condition of the patient also affects the volume of intravenous solution to be administered. When the patient has impaired kidney function, you must be very careful not to overload the patient’s system.
2-13. ENZYMES
An enzyme is a complex biological catalyst. Most reactions, which are aided by catalysts in the body, would take place without the enzyme, but too slowly to support life. Enzymes speed up chemical reactions during which complex substances are broken down into simple substances. The enzymes also speed the assembly of simple substances into complex substances. The enzyme is a protein that is not consumed or used up during its action as a catalyst. In the presence of an enzyme, a reaction uses up less energy for its completion. Most enzymes have the suffix “ase” in their name. This suffix is combined with the chemical name of the substance that uses the enzyme as a catalyst. Enzymes that split starch (amylum) are called “amylase” and those that react with fat (lipid) are called “lipase.” Most others are named in a similar manner.
2-14. HORMONES
Hormones are substances that are secreted by a group of glands in the body. This collection of glands is called the endocrine system. The hormones are carried by the blood to places in the body where they regulate the functions of their “target” organs. These hormones tell the organs when to operate and regulate their operation rate. The hormones coordinate body activities, control growth and development, and maintain homeostasis. When the supplying glands produce too few hormones (or stop producing), the body functions are always affected. An example of this is the insulin produced by a part of the pancreas. When the production of this hormone is insufficient, the body develops the disease we call “diabetes mellitus,” an inability to properly oxidize carbohydrates.